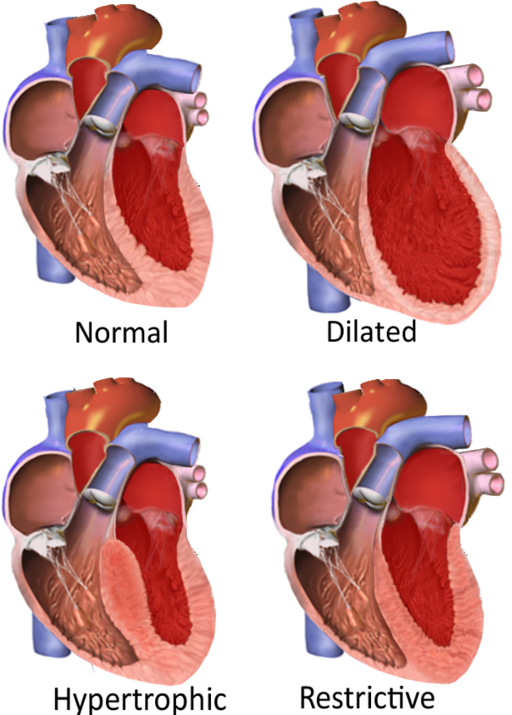
The NCCPA™ PANCE Cardiology System Content Blueprint includes 3 different types of cardiomyopathy
| Cardiomyopathy translates to “heart muscle disease,” so cardiomyopathy is a broad term used to describe a variety of issues that result from disease of the myocardium, or heart muscle. | |
Types of cardiomyopathies
|
| Impaired Systolic Function | |
| Dilated Cardiomyopathy | Patient will present as → a 68-year-old patient who comes to the office because of increased shortness of breath for four months. His symptoms are particularly bad at night. Medical history includes long-standing hypertension and alcoholism. Examination shows a displaced apex beat and normal breath sounds. Cardiac auscultation shows an S3 gallop and a pan-systolic murmur radiating to the axilla. The chest X-ray shows an enlarged left ventricular shadow. Dilated cardiomyopathy is the most common type (95%) of cardiomyopathy and is a condition in which an index event or process (such as an MI) damages the myocardium, weakening the heart muscle resulting in reduced strength of ventricular contraction, and dilation of the left ventricle
"An S3 gallop signifies the end of rapid ventricular filling in the setting of fluid overload and is often associated with dilated cardiomyopathy." DX: Echocardiography is the most definitive diagnosis - demonstrates left ventricular dilation and dysfunction and low cardiac output with poor EF (< 50%, but often less than 30%)
TX: βblocker + ACE + Loop Diuretic
|
| Impaired Diastolic Function | |
| Hypertrophic Obstructive Cardiomyopathy (HOCM) | Patient will present as → a 25-year-old man is brought to the ED because he collapsed while playing tennis 20 minutes ago. Medical history includes unexplained chest pain and shortness of breath while exercising for three years. Family history includes an uncle who died of an unknown cardiac pathology at the age of 23. Cardiac auscultation shows a 2/6 systolic murmur is heard at the left of the sternum between the first two ribs. The murmur becomes louder when the patient performs a Valsalva maneuver and decreases with squatting. The hypertrophic portion of the septum - LV outflow tract is narrowed - during systole, and obstruction worsened with increased contractility Presentation: A young athlete with a positive family history has a sudden death or syncopal episode
Physical Exam:
"The murmur due to HCM will increase in intensity with any maneuver that decreases the volume of blood in the left ventricle (such as standing abruptly or the strain phase of a Valsalva maneuver ). " DX: Diagnosis is by echocardiography or MRI
TX: β-blockers (metoprolol) and/or rate-limiting Ca channel blockers (usually verapamil) to decrease myocardial contractility and slow the heart rate and thus prolong diastolic filling and decrease outflow obstruction
|
| Restrictive Cardiomyopathy | Patient will present as → a 58-year-old man complaining of several months of worsening shortness of breath and ankle swelling. He denies palpitations, lightheadedness, syncope, or chest pain. He has a past medical history significant for hereditary hemochromatosis. On physical exam, his temperature is 37 C (98.6 F), pulse is 78, blood pressure is 130/72 mm Hg, and respiratory rate is 16. He has elevated jugular venous pressure, diminished breath sounds at the lung bases, tender hepatomegaly, and bilateral pitting ankle edema. There are no murmurs, rubs, or gallops. EKG shows low-voltage QRS complexes without any signs of ischemia. His chest x-ray shows a normal-sized heart and bilateral pleural effusions. Echocardiography shows symmetrical thickening of the left ventricle, normal left ventricular volume, and mildly reduced systolic function. Right heart failure with a history of an infiltrative process
DX: Echocardiography shows a normal left ventricular ejection fraction. Common findings include dilated atria and myocardial hypertrophy
TX: often unsatisfactory unless the cause can be addressed
|