NCCPA™ PANCE Endocrine System Blueprint diabetes mellitus type I and type II (PEARLS)
| Diabetes mellitus type 1
Patient will present as → a young patient with weight loss, increased thirst, and urination. The patient has felt tired and nauseous. On examination, her weight is below the 5th percentile, she looks thin, and her skin is pale. Her blood pressure is 100/70, and her pulse is 104 bpm. Her respirations are deep at a rate of 28 breaths/minute. Her breath smells fruity Etiology: Autoimmune - HLA-DR3/4/O antibodies. Islet cell antibodies Presentation of Type I DM:
Treatment of Type I DM: Insulin Dawn Phenomenon: Normal glucose until 2-8 am when it rises. Results from decreased insulin sensitivity and a nightly surge of counter-regulatory hormones during nighttime fasting
Somogyi effect: Nocturnal hypoglycemia followed by rebound hyperglycemia due to a surge in growth hormone
Check 3 am blood sugar!
Insulin waning: a progressive rise in glucose from bedtime to morning DKA: Fruity breath, weight loss, rapid respirations, hypotension
Diagnosis of DM is made by one of the following:
Insulin and C-peptide levels – low or inappropriately normal fasting C-peptide and insulin levels with concomitant hyperglycemia
Insulin, GAD65, and IA-2 antibodies ⇒ if one or more of the antibodies is present, and especially if two or more are positive, the patient should be presumed to have type 1 diabetes and should be treated with insulin replacement therapy Monitoring/evaluation of glycemic control
Blood pressure management
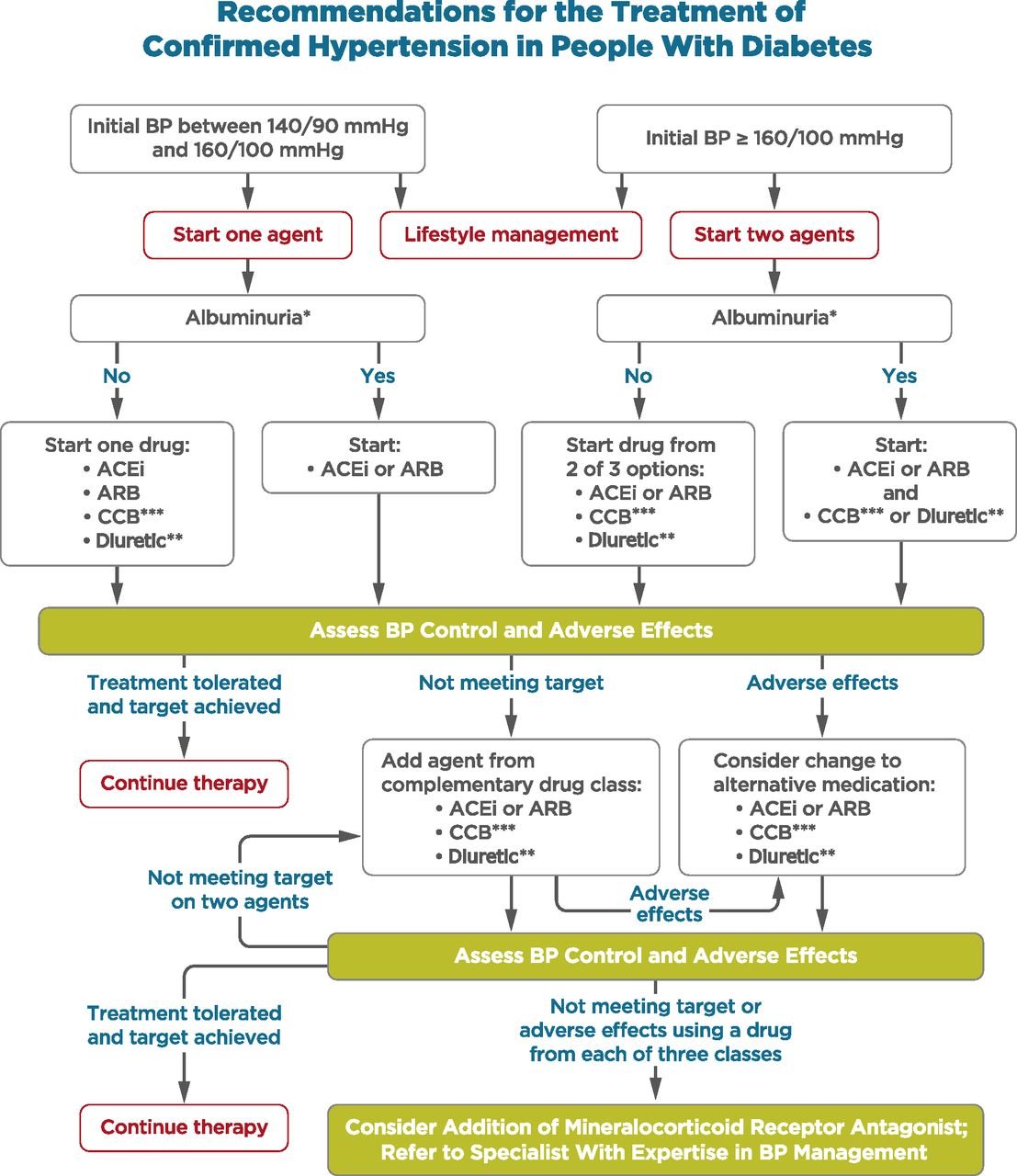 From ADA Standards - Section 10, CV Disease **Thiazide-like diuretic; long-acting agents shown to reduce cardiovascular events, such as chlorthalidone and indapamide, are preferred. ***Dihydropyridine calcium channel blocker (CCB). |
| Insulins | ||||
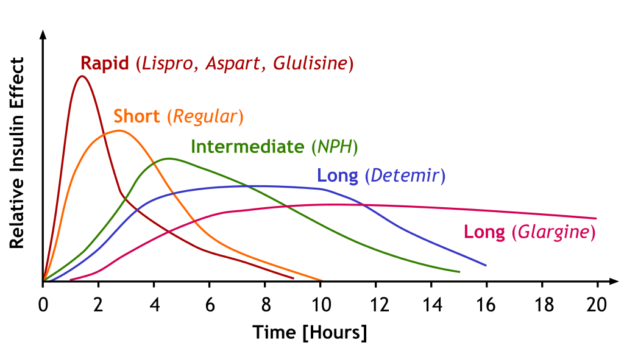 Image by A. Peters, M. Komorniczak. License: CC BY 3.0 |
||||
| Insulin effect | Type of Insulin | Onset of action | Peak of action | Duration of action |
| Fast acting | Lispro | 15–30 minutes | 1–3 hours | 4–6 hours |
| Aspart | ||||
| Glulisine | ||||
| Short-acting | Regular | 30 minutes | 1.5–3.5 hours | 8 hours |
| Intermediate-acting | NPH | 1–2 hours | 4–6 hours | > 12 hours |
| Long-acting | Detemir | 1–2 hours | 3–9 hours | 14–24 hours |
| Glargine | 3–4 hours | No peak | Approximately 24 hours | |
| Degludec | Approximately 1 hour | No peak | > 40 hours | |
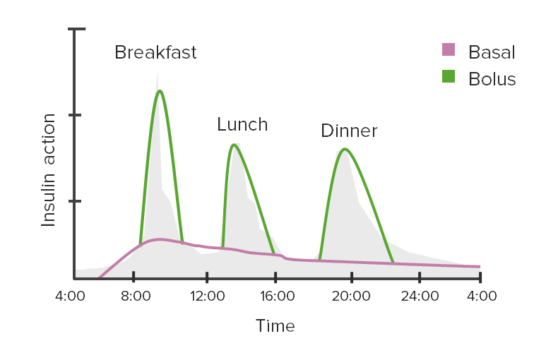
Example of insulin regimen:
|
||||
| Oral therapies | |||
| Intervention | Expected decrease in A1C with monotherapy (%) | Advantages | Disadvantages |
| Lifestyle changes to decrease weight and increase activity | 1.0 to 2.0 | Broad benefits | Insufficient for most within the first year owing to inadequate weight loss and weight regain |
| Metformin | 1.0 to 2.0 | Weight neutral | GI side effects, contraindicated with renal insufficiency (eGFR <30 mL/min) |
| Additional therapies | |||
| Insulin (usually with a single daily injection of intermediate- or long-acting insulin initially) | 1.5 to 3.5 | No dose limit, rapidly effective, improved lipid profile | One to four injections daily, monitoring, weight gain, hypoglycemia, analogues are expensive |
| Sulfonylurea (shorter-acting agents preferred) | 1.0 to 2.0 | Rapidly effective | Weight gain, hypoglycemia |
| GLP-1 agonist (daily to weekly injections) | 0.5 to 1.0 | Weight loss and reduced cardiovascular mortality (liraglutide, semaglutide) in patients with established CVD | Requires injection, frequent GI side effects, long-term safety not established, expensive |
| Thiazolidinedione | 0.5 to 1.4 | Improved lipid profile (pioglitazone), potential decrease in MI (pioglitazone) | Fluid retention, HF, weight gain, bone fractures, potential increase in MI (rosiglitazone) and bladder cancer (pioglitazone) |
| Meglitinides | 0.5 to 1.5 | Rapidly effective | Weight gain, three times/day dosing, hypoglycemia |
| SGLT2 inhibitor | 0.5 to 0.7 | Weight loss, reduction in systolic blood pressure, reduced cardiovascular mortality in patients with established | Vulvovaginal candidiasis, urinary tract infections, bone fractures, lower limb amputations, acute kidney injury, DKA, long-term safety not established |
| DPP-4 inhibitor | 0.5 to 0.8 | Weight neutral | Long-term safety not established, expensive, possible increased risk of HF with saxagliptin |
| Alpha-glucosidase inhibitor | 0.5 to 0.8 | Weight neutral | Frequent GI side effects, three times/day dosing |
| Pramlintide | 0.5 to 1.0 | Weight loss | Three injections daily, frequent GI side effects, long-term safety not established, expensive |
| Table source: UpToDate | |||
Monitoring in patients with diabetes mellitus (summary table)
| History and physical examination | ||
| Height, weight, and BMI | Every visit | |
| Smoking cessation counseling | Every visit | For smokers only. |
| Blood pressure | Every visit | Goal systolic pressure 125 to 130 mmHg. |
| Dilated eye examination | Annually | Begin at onset of type 2 diabetes, 3 to 5 years after onset of type 1 diabetes. Examine yearly (or more frequently) if retinopathy is present, every 2 to 3 years if there is no evidence of retinopathy. |
| Comprehensive foot examination | Annually | Every visit if peripheral vascular disease or neuropathy. |
| Dental examination | Annually | Periodontal disease is more severe but not necessarily more prevalent in patients with diabetes. |
| Laboratory studies | ||
| Lipid profile | Initially, as indicated | In people without dyslipidemia and not on cholesterol-lowering therapy, testing may be infrequent. |
| A1C | Every 3 to 6 months | Goal ≤ 7% (may be lower or higher in selected patients). |
| Urinary albumin-to-creatinine ratio | Annually | Begin 3 to 5 years after onset of type 1 diabetes and at diagnosis in patients with type 2 diabetes; protein excretion should also be monitored if persistent albuminuria is present. |
| Serum creatinine | Initially, as indicated | Typically annually; more often in the presence of chronic kidney disease. |
| Vaccinations | ||
| Pneumococcus | ||
|
1 dose, ages 19 to 64 years | Once the patient is ≥ 65 years (and ≥1 year after PCV13 and > 5 years after previous dose of PPSV23), give a second dose of PPSV23. Revaccinate every 10 years. |
|
1 dose at age ≥ 65 years | Once the patient is ≥65 years (and ≥1 year after PPSV23), give PCV13. |
|
1 dose, ages 19 to 64 years or ≥ 65 years if no prior dose. No additional pneumococcal vaccine is needed! | |
| Influenza | Annually | |
| Hepatitis B | 3-dose series | Administer to unvaccinated adults who are ages 19 to 59 years. For older patients, administer based upon risk of acquiring hepatitis B, including the need for assisted blood glucose monitoring and the likelihood of an adequate immune response to vaccination. |
| Provide other routine vaccinations for adults with diabetes according to age-related recommendations. | ||
| Education, self-management review | ||
| Annually | More often at onset of diabetes and when there is a change in regimen. | |
| Diabetes Mellitus Type 2 |
ReelDx Virtual Rounds (Diabetes Mellitus Type 2)Patient will present as → a 35-year-old Mexican American male complaining of increased thirst, frequent urination, hunger, fatigue, and blurred vision random finger stick blood glucose is 225. Diagnosis: random glucose > 200 x two or fasting glucose > 126 x two, A1c of > 6.5% Diabetes Medications: Metformin - decreases hepatic glucose production and peripheral glucose utilization, decreases intestinal glucose absorption (these are reasons it leads to weight loss)
Sulfonylureas - stimulates pancreatic beta-cell insulin release (insulin secretagogue)
Thiazolidinediones - increases insulin sensitivity in peripheral receptor site adipose and muscle has no effect on pancreatic beta cells
Alpha-glucosidase inhibitors - Delays intestinal glucose absorption
Meglitinides - stimulates pancreatic beta-cell insulin release
GLP-1 Agonists - lowers blood sugar by mimicking incretin - causes insulin secretion and decreased glucagon and delays gastric emptying
DPP-4 Inhibitors - dipeptidyl peptidase inhibition - inhibits degradation of GLP-1 so more circulating GLP-1
SGLT2 Inhibitor - SGLT2 inhibition lowers renal glucose threshold which results in increased urinary glucose excretion
Insulin – add if HbA1C > 9 Follow Up: Annual- ophthalmologist visit, urine microalbumin Complications – neuropathy (most common), retinopathy (the leading cause of blindness), nephropathy Normal fasting glucose is between 70 and 100 Diagnosis of DM is made by one of the following:
Diagnostic criteria for prediabetes
Glucose goals and basic management
|
| Hyperosmolar hyperglycemic syndrome/state (HHS)
Patient will present as → a 72-year-old female with poorly controlled type 2 diabetes presents to the ED with altered mental status, lethargy, decreased oral intake, polyuria, polydipsia, and blurred vision. She is disoriented, severely dehydrated, and mildly acidotic. Labs reveal a blood glucose level of 900 mg/dL, serum osmolality of 320 mOsm/kg, and negative ketones. The patient is admitted to the ICU for IV fluids, insulin therapy, and electrolyte replacement. A presumed UTI is treated with antibiotics. She improves with treatment, transitions to subcutaneous insulin, receives diabetes education, and her medication regimen is adjusted. Hyperosmolar hyperglycemic syndrome/state (HHS) is a serious complication of type 2 diabetes characterized by severe hyperglycemia, extreme dehydration, high blood osmolarity, and altered consciousness, often triggered by physiologic stress Think '3 Hs': Hyperglycemia + Hyperosmolarity + Hydration (severe dehydration)
DX: HHS is suspected with significantly elevated blood glucose levels found during the evaluation of altered mental status. A fingerstick glucose test often serves as the first indicator Key Diagnostic Tests:
TX: HHS requires immediate hospitalization. Treatment focuses on:
Complications: Can lead to coma, seizures, and death if not promptly treated, with a mortality rate up to 20% |
The dawn phenomenon is the presence of hyperglycemia upon awakening. The increase in blood glucose is caused by increased hormone production in the early morning hours. Between the hours 2AM-6AM, the body naturally increases production of growth hormone and cortisol. Treatment involves changing the patient’s current insulin regimen. The patient may have their current administration times changed, be given a long-acting insulin in the evening, or be given an insulin pump. Since insulin affects blood glucose levels, the patient’s glucose levels should be closely monitored particularly between 2AM-6AM. Instruct the patient to limit carbohydrates before bedtime to avoid spikes in blood sugar during the night.
Play Video + QuizDiabetic ketoacidosis (DKA)
Diabetic ketoacidosis (DKA) is a medical emergency and complication of diabetes. Patients have increased insulin requirements, which leads to a shortage. As a response, the body begins burning excess fat (and fatty acids), causing ketone body buildup. Lab values seen in DKA include blood sugars above 250 mg/dL, and anion gap metabolic acidosis with pH below 7.3 and bicarbonate below 18. Patients will also show present plasma ketones. Due to an extracellular shift, patients may be hyperkalemic.
| Diabetic Ketoacidosis (DKA) Treatment | Play Video + Quiz |
| Diabetic Ketoacidosis (DKA) Signs and Symptoms | Play Video + Quiz |
| Diabetic Ketoacidosis (DKA) Diagnosis and Labs | Play Video + Quiz |
Diabetic glomerulonephropathy
Diabetic glomerulonephropathy is the kidney disease seen in patients with significantly progressed diabetes. There is damage to the kidney due to nonenzymatic glycosylation of the basement membrane which alters the permeability of arterioles and glomeruli and results in nephrotic syndrome. Patients will have increased GFR due to preferential efferent sclerosis, mesangial expansion due to hyperfiltration pressure, and will ultimately enter kidney failure if glucose levels are not controlled.
Play Video + Quiz

 Picmonic
Picmonic