Patient will present as → a 56-year-old male two days post–bilateral ureterosigmoidostomy for bladder resection due to cancer. He complains of increasing shortness of breath. The patient denies cough, chest pain, or fever. Physical examination is unremarkable except for an increased respiratory rate of 30 breaths/min. Arterial blood gas reveals pH of 7.28, pCO2 22 mmHg, and HCO3 13 mEq/L
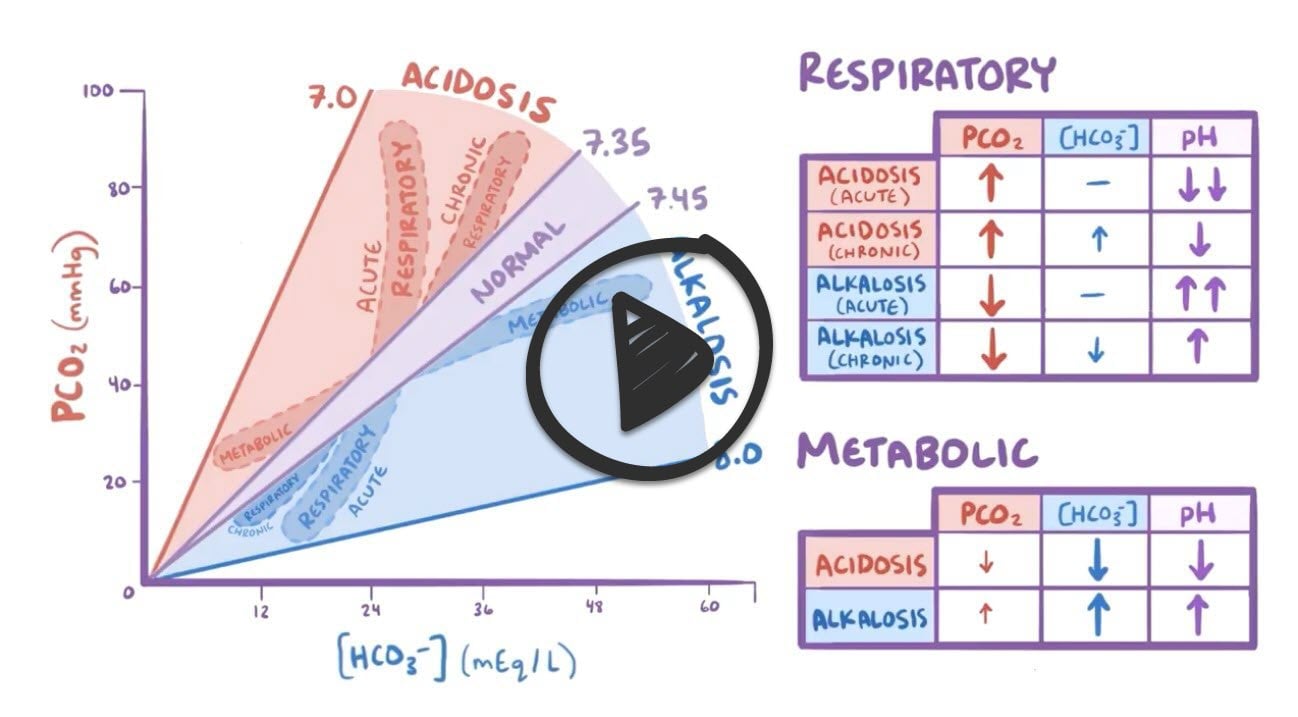
Average values "24/7 40/40"
- 24 (HCO3, base) / 7.40 (pH) / 40 (CO2, acid)
The case in the presentation represents metabolic acidosis with a low PH 7.2 (acidosis) and abnormal HCO3
Three Step Approach to Acid Base Disorders
- Look at your PH (7.35-7.45 is normal)
- < 7.35 = acidosis
- > 7.45 = alkalosis
- Next look at your PCO2 is it normal low or high (35-45 normal)
- ↑ CO2 and ↓PH = respiratory acidosis
- ↓ CO2 and ↑ PH = respiratory alkalosis
- If you don’t see a change in the CO2 in relation to the PH then take a look at the HCO3
- Finally, look at the HCO3 is it normal low or high (20-26 normal)
- ↓ HCO3 and ↓PH = metabolic acidosis
- ↑ HCO3 and ↑ PH =metabolic alkalosis
Table comparing types of acid-base disorders:
| Type | Example | Cause |
| Respiratory Acidosis | PH 7.30, high PCO2 60, Normal Bicarb 22 | Lungs fail to excrete CO2 (Breathing too slow (holding onto CO2), pulmonary disease, neuromuscular disease, drug-induced hypoventilation - opiates, barbiturates) |
| Respiratory Alkalosis | PH 7.52, low PCO2 25, Normal Bicarb 22 | Excessive elimination of CO2 (Breathing too fast (blowing of CO2), pulmonary embolism, fever, hyperthyroid, anxiety, salicylate intoxication, septicemia ) |
| Metabolic Acidosis | PH 7.30, Normal PCO2 40, Low Bicarb 16 | Need to calculate anion gap: Anion Gap = Na – (Cl + HCO3-) = 10-16
Increased ion gap (>16): Addition of hydrogen ions (lactic acidosis (think metformin), diabetic ketoacidosis, aspirin overdose)
Low anion gap (<16): Loss of bicarbonate (think diarrhea, pancreatic or biliary drainage, renal tubular acidosis) |
| Metabolic Alkalosis | PH 7.52, Normal PCO2 40, High Bicarb | Loss of hydrogen (vomiting), bulimia, overdose of antacids, addition of bicarbonate (hyperalimentation therapy) |
 Osmosis Osmosis |
|
 |
 Respiratory acidosis is a medical condition characterized by decreased ventilation, which causes increased levels of carbon dioxide in the blood (PCO2 > 40) leading to a decrease in blood pH. Carbon dioxide is constantly produced via metabolic reactions in the body that is efficiently expelled through the lungs during alveolar ventilation. Common causes of decreased alveolar ventilation include depression of the central respiratory center by sedatives like barbiturates or opioids, airway obstruction including asthma or COPD exacerbations, or neuromuscular disorders that cause respiratory muscle weakness or paralysis.
Respiratory acidosis is a medical condition characterized by decreased ventilation, which causes increased levels of carbon dioxide in the blood (PCO2 > 40) leading to a decrease in blood pH. Carbon dioxide is constantly produced via metabolic reactions in the body that is efficiently expelled through the lungs during alveolar ventilation. Common causes of decreased alveolar ventilation include depression of the central respiratory center by sedatives like barbiturates or opioids, airway obstruction including asthma or COPD exacerbations, or neuromuscular disorders that cause respiratory muscle weakness or paralysis.
| Respiratory Acidosis | Play Video + Quiz |
| Respiratory Acidosis Interventions | Play Video + Quiz |
Respiratory alkalosis
 Respiratory alkalosis is an acid-base imbalance marked by decreased levels of blood carbon dioxide with subsequent pH elevation. The direct cause is an increase in respiratory rate, which results in the excessive loss of CO2 on exhalation. The cause of increased respiratory rate is quite variable including high altitude, aspirin toxicity, restrictive lung disease, pulmonary embolism, pregnancy or anxiety. Carbon dioxide is an acidic molecule which helps maintain the blood’s pH close to 7.4. When it is excessively exhaled, this balance is disrupted leading to an increase in the blood pH. This is referred to as respiratory alkalosis.
Respiratory alkalosis is an acid-base imbalance marked by decreased levels of blood carbon dioxide with subsequent pH elevation. The direct cause is an increase in respiratory rate, which results in the excessive loss of CO2 on exhalation. The cause of increased respiratory rate is quite variable including high altitude, aspirin toxicity, restrictive lung disease, pulmonary embolism, pregnancy or anxiety. Carbon dioxide is an acidic molecule which helps maintain the blood’s pH close to 7.4. When it is excessively exhaled, this balance is disrupted leading to an increase in the blood pH. This is referred to as respiratory alkalosis.
| Respiratory Alkalosis | Play Video + Quiz |
| Respiratory Alkalosis Interventions | Play Video + Quiz |
Normal Gap Metabolic Acidosis
Normal gap metabolic acidosis occurs when the body’s pH drops below 7.4 but a normal anion gap is maintained between the sodium, chloride and bicarbonate concentrations. The calculation is made by subtracting the chloride and bicarbonate concentrations from the sodium concentration and seeing a value less than 11. This signifies that there is no other anion causing an acidosis. This type of metabolic acidosis is sometimes referred to as a hyperchloremic metabolic acidosis, described by either an increase in plasma Cl– or a decrease in plasma bicarbonate. The common causes of normal gap acidosis can be remembered with the acronym HARD-ASS: hyperalimentation, Addison’s disease, renal tubular acidosis, diarrhea, acetazolamide, spironolactone, or saline infusion.
Play Video + QuizAnion gap metabolic acidosis
Anion gap metabolic acidosis is a metabolic state in which the body’s pH drops below its physiologic level. This is due to the addition of an acid to the blood. Recall from general chemistry that many acids have a low pKa, and therefore will largely exist in deprotonated or anionic form when in solution near body pH. The end result is the addition of an acid (H) to the blood, as well as its corresponding anion. This unmeasured anion makes the anion gap larger. The common causes of this abnormality are methanol, uremia, ketoacidosis, propylene glycol, iron toxicity, isoniazid, lactic acidosis, ethylene glycol, and salicylate (aspirin) toxicity.
Play Video + QuizMetabolic Alkalosis
 Metabolic alkalosis is a metabolic state where the body’s pH is elevated due to increased bicarbonate concentrations. The elevations in bicarbonate can be due to decreased bicarbonate excretion by the kidney, increased bicarbonate intake, or volume depletion. The most common causes are diuretics, vomiting, antacids, and hyperaldosteronism
Metabolic alkalosis is a metabolic state where the body’s pH is elevated due to increased bicarbonate concentrations. The elevations in bicarbonate can be due to decreased bicarbonate excretion by the kidney, increased bicarbonate intake, or volume depletion. The most common causes are diuretics, vomiting, antacids, and hyperaldosteronism
| Metabolic Alkalosis Picmonic | Play Video + Quiz |
| Metabolic Alkalosis Interventions Picmonic | Play Video + Quiz |
Winter’s Formula
Winter’s Formula is used to evaluate respiratory compensation when metabolic acidosis is present in a patient. This is used to give an expected value for the patient’s PCO2, which helps to assess whether or not the patient is adequately compensating for their acidotic state. Winter’s formula yields the expected PCO2 = (HCO3 x 1.5) + 8 ± 2.
Play Video + QuizQuestion 1 |
Respiratory acidosis | |
Respiratory alkalosis | |
Metabolic acidosis | |
Metabolic alkalosis |
Question 2 |
Respiratory acidosis | |
Respiratory alkalosis | |
Metabolic acidosis | |
Metabolic alkalosis |
Question 3 |
Respiratory acidosis | |
Respiratory alkalosis | |
Metabolic acidosis | |
Metabolic alkalosis |
Question 4 |
Respiratory acidosis | |
Respiratory alkalosis | |
Metabolic acidosis | |
Metabolic alkalosis |
|
List |





